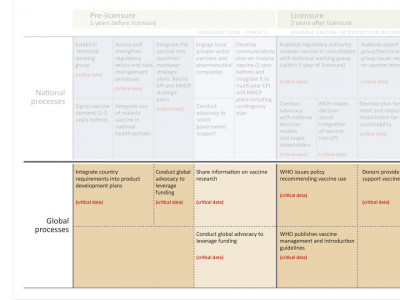
Global level processes appear in brown boxes, also arranged by time period. For example, pharmaceutical manufacturers will need to integrate the countries’ requirements for safety, efficacy, and other vaccine characteristics into their product development plans five years before licensure and prior to Phase 3 clinical trials.
Download the planning tool (1.6 MB PDF)
